Natural Garlic Oil – Radiocarbon Carbon-14 (14C) Test
What is Carbon-14 (14C) Radiocarbon
Carbon-14 (14C), or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz Kurie in 1934.[2]
There are three naturally occurring isotopes of carbon on Earth: carbon-12, which makes up 99% of all carbon on Earth; carbon-13, which makes up 1%; and carbon-14, which occurs in trace amounts, making up about 1 or 1.5 atoms per 1012 atoms of carbon in the atmosphere. Carbon-12 and carbon-13 are both stable, while carbon-14 is unstable and has a half-life of 5,730 ± 40 years.[3] Carbon-14 decays into nitrogen-14 through beta decay.[4] A gram of carbon containing 1 atom of carbon-14 per 1012 atoms will emit ~0.2[5] beta particles per second. The primary natural source of carbon-14 on Earth is cosmic ray action on nitrogen in the atmosphere, and it is therefore a cosmogenic nuclide. However, open-air nuclear testing between 1955 and 1980 contributed to this pool.
The different isotopes of carbon do not differ appreciably in their chemical properties. This resemblance is used in chemical and biological research, in a technique called carbon labeling: carbon-14 atoms can be used to replace nonradioactive carbon, in order to trace chemical and biochemical reactions involving carbon atoms from any given organic compound.
References
- Waptstra, A.H.; Audi, G.; Thibault, C. “AME atomic mass evaluation 2003”. Archived from the original on 2008-09-23. Retrieved 2007-06-03.
- ^ Kamen, Martin D. (1963). “Early History of Carbon-14: Discovery of this supremely important tracer was expected in the physical sense but not in the chemical sense”. Science. 140(3567): 584–90. Bibcode:1963Sci…140..584K. doi:10.1126/science.140.3567.584. PMID 17737092.
- ^ Godwin, H. (1962). “Half-life of radiocarbon”. Nature. 195(4845): 984. Bibcode:1962Natur.195..984G. doi:10.1038/195984a0. S2CID 27534222.
- ^ “What is carbon dating?”. National Ocean Sciences Accelerator Mass Spectrometry Facility. Archived from the original on July 5, 2007. Retrieved 2007-06-11.
- ^ (1 per 1012) × (1 gram / (12 grams per mole)) × (Avogadro constant) / ((5,730 years) × (31,557,600 seconds per Julian year) / ln(2))
Garlic Oil natural purity is always the focus and priority by many food companies and trading companies. Therefore, when Hefei Dielegance Biotechnology Co., Ltd. launches the plan to initiating research and development on garlic oil, we are dedicated to the research on the naturality and purity of garlic oil. Due to the advanced production equipment, test equipment and strict QC, our garlic oil quality always represents the world leading level. In the next half year of 2021, we send another batch sample for natural purity test in response to our customers.


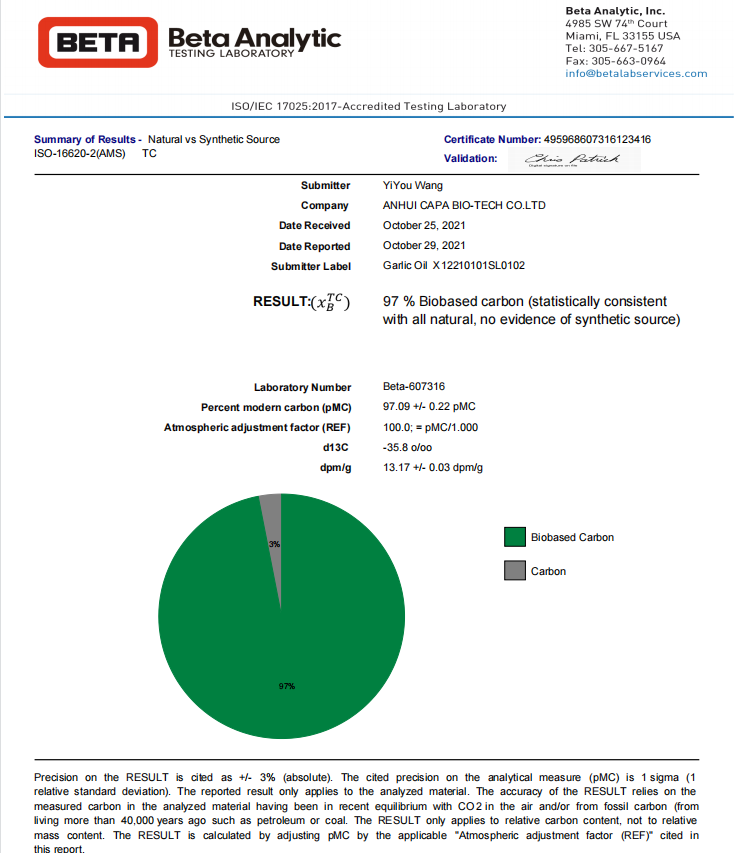


Hefei Dielegance Biotechnology Co., Ltd. is always on the way to produce and supply industry-leading quality natural garlic oil for the customers around the world. Based on the competitive price and quality consistency, food companies and pharm companies as well as healthcare supplement companies around the world has established long-term solid business relationship with us.
If you are looking for natural garlic oil, Dielegance Biotechnology is your trustworthy business partner. For more product details, you can check it in our product page Garlic Oil. Welcome to contact us or send us an email for inquiry.